What we know about the COVID-19 vaccine
December 10, 2020
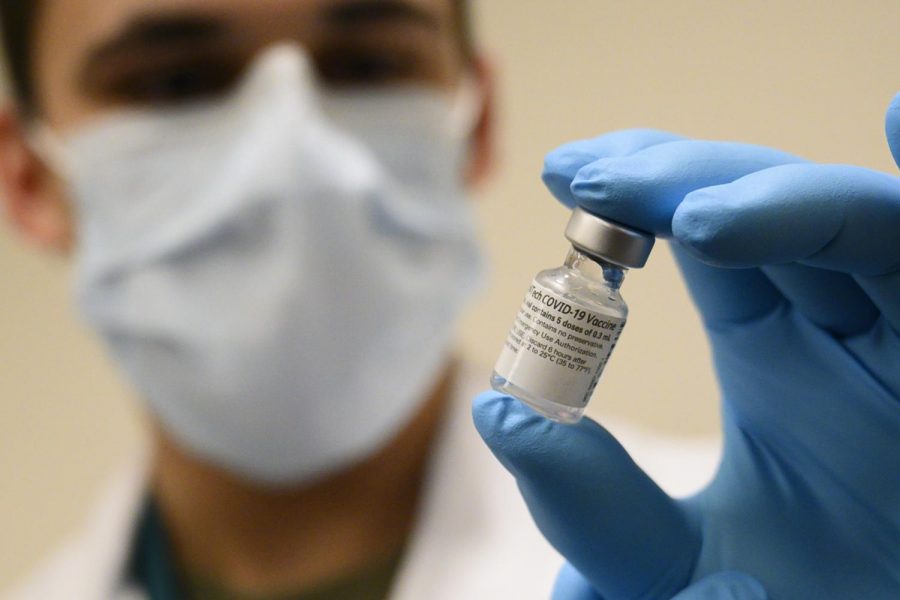
After nearly 10 months of research and trials, the American pharmaceutical giant Pfizer has developed a potential vaccine to protect against COVID-19, making it the fastest created vaccine in history. Now, with a potential solution to the worldwide pandemic, information and understanding is more important than ever.
Pfizer and their German partner company BioNTech tested around 44,000 participants in clinical trials, giving half of their participants the vaccine and the other half a placebo. According to Pfizer, the experimental vaccine is 95% effective against COVID-19, including those who are more at risk.
The BNT162b2 vaccine uses RNA, the genetic material that cells read to develop proteins, and mRNA as a protective barrier for said RNA. Once injected the vaccine particles release mRNA after fusing to the cells. The fusing of the vaccine particles and the cells trigger the cell molecules to start reading the sequence, creating spike proteins. The vaccinated cells will then break up some of the proteins into fragments, so they can be recognized by the body’s immune system as intrusive. According to Uğur Şahin, one of the founders of BioNTech and the brains behind BNT162b2, the vaccine trains specific antibodies to fight off the virus. The antibody cells B and T learn to attack invading pathogens as preparation for another encounter with COVID-19 in the system, creating an immune memory.
For the immunity to stick, the vaccine requires two doses. Şahin estimated that the BNT162b2 vaccine will be effective for months, or even years and will most likely become an annual inoculation.
Now that Pfizer has established the effectiveness of the BNT162b2 vaccine after months of experimentation and trials, the company is seeking an Emergency Use Authorization from the Food and Drug Administration and working towards making the vaccine available to the public. On Dec. 2, the U.K. granted Pfizer the requested authorization, becoming the first western country to authorize the use of a COVID-19 vaccine. The U.K. had pre-ordered 40 million vaccines and has been working on a countrywide rollout plan to vaccinate the most vulnerable in the population first, as reported by The New York Times. Now, with the U.K. getting aggressive with their efforts to protect people against the virus, the pressure is on U.S. officials to follow suit. The F.D.A plans to discuss the idea of authorizing the use of the vaccine, and due to the somewhat controversial nature of the topic, it could be a while until the members come to a consensus
There is still quite a bit of skepticism surrounding the BNT162b2 vaccines and similar vaccines currently being tested, mainly in regards to potential side effects. Drug companies have done their best to be transparent about the side effects, such as soreness around the area and fatigue, but the possibility of that information being misconstrued could be worrisome.